Chemistry Practicals Class 10
Neutralization Reaction
- Teach science experiments in a gamified way
- Boost conceptual clarity and knowledge retention
- Aligned with National Education Policy 2020
- Helpful in getting NAAC accreditation
- CBSE, ICSE, and state boards aligned curricula
- Engaging simulations with easy-to-teach instructions
About Simulation
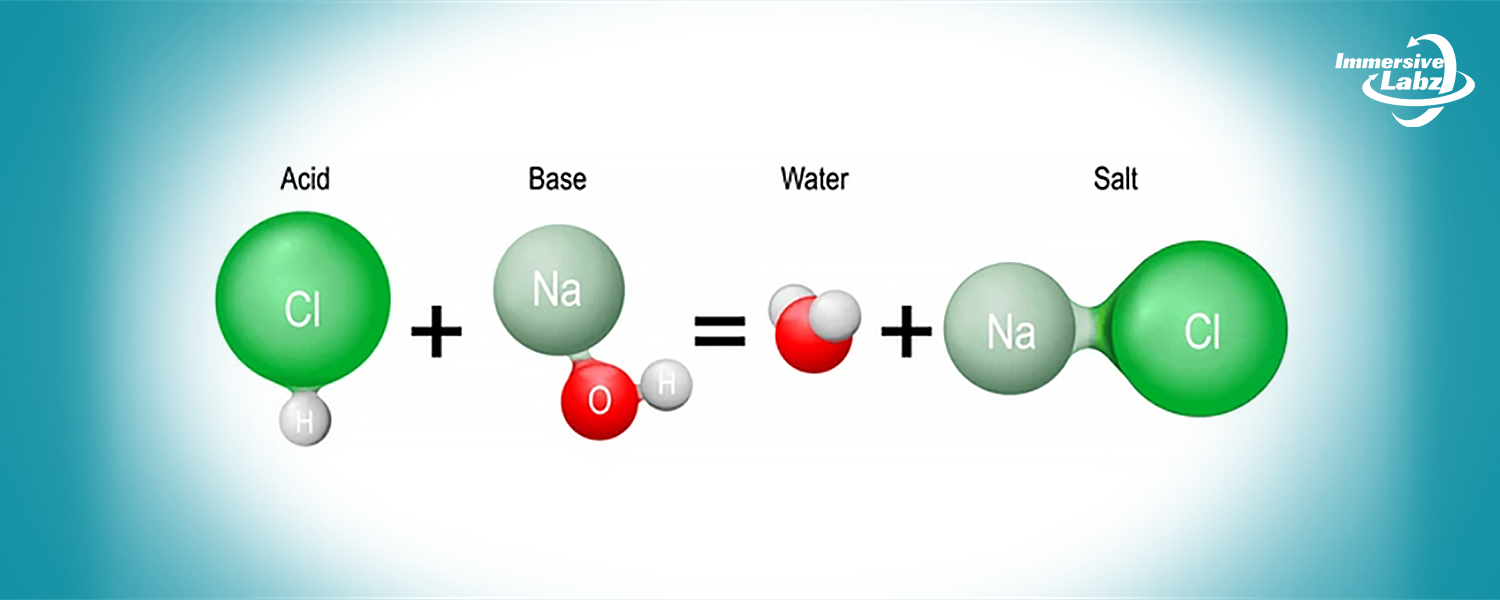
- You will have access to a virtual laboratory setting through the simulation for the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH).
- Through this simulation, you can deepen your understanding of the concept of neutralization reactions involving acids and bases in chemistry.
- By engaging with the simulation, you can observe firsthand and analyze the interaction between acids and bases during the neutralization process, enhancing your comprehension of chemical reactions.
- You can explore the progression of the neutralization reaction between hydrochloric acid and sodium hydroxide within the simulation, providing insights into the balance of ions and the formation of water and salt.
- Through this virtual science experiment, you will gain valuable knowledge about the principles underlying neutralization reactions, which are fundamental in understanding acid-base chemistry and its applications.
Simulation Details

Duration – 30 Minutes

Easily Accessible

Languages – Odia & English

Platforms – Android & Windows
Description
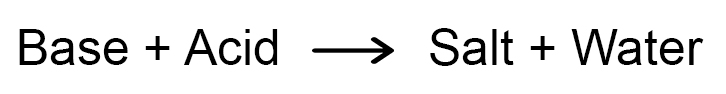
Neutralization Reaction:
The reaction between an acid and a base to give salt and water is known as a neutralization reaction. In general, a neutralization reaction can be written as –
Components Involved:
- Acid: Acids are substances that release hydrogen ions (H+) in aqueous solutions. Common examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and citric acid found in citrus fruits.
- Base: Bases are substances that release hydroxide ions (OH–) in aqueous solutions. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
Acid-Base Neutralization Reaction:
- In the neutralization reaction between HCl and NaOH, hydrogen ions (H+) from the acid combine with hydroxide ions (OH–) from the base.
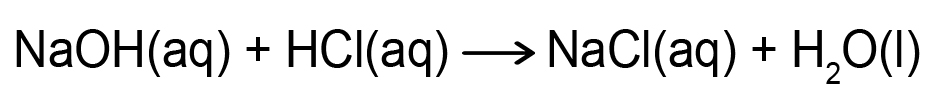
Watch this video to learn more about chemistry.
Requirements for this Science Experiment
- Hydrochloric acid
- Sodium hydroxide solution
- Test tube
- Test tube stand
- Droppers
- Phenolphthalein indicator
Why Choose SimuLab for Science Practicals?


Try SimuLab
A 3D virtual science lab (physics lab, chemistry lab, and biology lab) that helps students learn science experiments easily.



