When two or more substances are physically combined, an impure substance is formed, known as a mixture. A two-phase heterogeneous system consists of one dispersed phase, the dispersion medium, and the particle size between 1–100 nm. Such a system is known as a colloidal system. The dispersed phase is a component present in a small proportion. The dispersion medium is present in large proportion in the system. The stable colloid is one in which the dispersed phase remains suspended throughout the dispersion medium.
For example, in a soil-water colloidal solution, the soil is the dispersed phase, and water is the dispersion medium. Based on the physical states of the dispersed phase and dispersion medium, colloids are further classified as sols (colloidal sols), in which the dispersed phase is solid, and the dispersion medium is liquid.
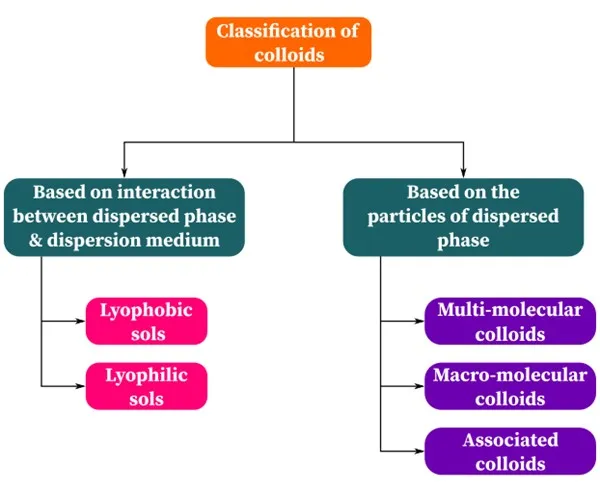
Lyophilic and lyophobic sols are the two types of sols, classified based on the nature of interactions between the dispersed phase and dispersion medium.
What are lyophilic sols?
The word ‘lyophilic’ suggests ‘liquid loving’ or ‘solvent loving’, also known as intrinsic colloids. In this sol, there is a strong affinity between the dispersed phase and dispersion medium due to the formation of a large number of hydrogen bonds. These sols are prepared directly by mixing substances like gum, starch, etc., with a suitable dispersion medium (liquid).
- The dispersed phase can be separated in any way from the dispersion medium. Original sol can be reconstituted by simply remixing the dispersed phase with a dispersion medium.
- The viscosity of the colloid formed is high.
- The charges on lyophilic sol are positive, negative and neutral.
- Egg albumin sol: The lyophilic sol is formed when the egg albumin is mixed with cold water. The sol obtained is stable and cannot be affected by the traces of impurities.
- Starch sol: Starch is added to the water, and the mixture is heated at 100 degree Celcius.
- Gum sol: Gum is quite stable in warm water; thus, the sol is prepared using warm water as a dispersion medium.
Some other examples of lyophilic sols are rubber, gelatine, and blood.
What are lyophobic sols?
The word ‘lyophobic’ suggests ‘liquid hating’ or ‘solvent hating’. The metals, their sulphides and hydroxides in a suitable dispersion medium form lyophobic sol. These sols cannot be prepared like lyophilic sols by mixing dispersed phases in the dispersion medium. Some special chemical and non-chemical methods are used for their preparation. Lyophobic sols are readily precipitated (or coagulated) by adding some reagent and electrolyte or by shaking and heating; hence, these sols are unstable.
- Lyophobic sols are irreversible.
- Stabilizing agents are added for the preservation of lyophobic sols.
- The charge on lyophobic sols can be positive or negative.
- Electrical disintegration, or Bredig’s arc method used for preparing colloidal sols of metals.
- The mechanical dispersion method is used to prepare sols of sulphur, indigo, printer ink, toothpaste, ointments, etc.
- Peptization: The precipitate is converted to colloids by shaking it with a suitable electrolyte (peptizing agent).
Disintegration or dispersion methods: Some physical techniques are utilized to reduce the size of colloidal particles, i.e., between 1 nm to 1000 nm.
Condensation methods:
Hydrolysis reaction: Iron hydroxide sol.
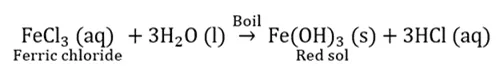
Double decomposition reaction: Arsenic sulphide sol.
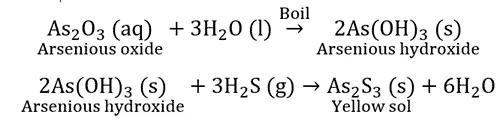
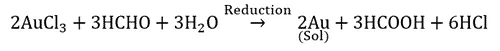
Reduction reaction: Metal sols like gold, silver and platinum.
Oxidation reaction: Colloid of sulphur
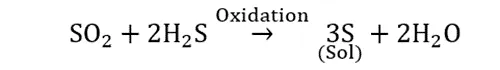
Non-chemical condensation methods are based on the exchange of solvent or condensing vapours (For example, colloid of sulphur).
Colloids prepared using different methods may contain impurities, suspended particles, and electrolytes. High concentration of impurities affects the stability of colloidal sols. Thus, it is essential to purity the sols during their preparation. Dialysis, electro-dialysis, ultrafiltration, ultra-centrifugation, etc., are various purification methods.
Conclusion:
Colloidal sols are a mixture of the dispersed phase (solid) and the dispersion medium (liquid). They are further divided into two types based on interactions between two phases. The reversible sols with strong interactions between the two phases are lyophilic, and irreversible sols with weaker interactions are lyophobic sols.
In comparison to the lyophobic sols, the preparation of lyophilic sol is easier. Simply heating, shaking or mixing two phases can form lyophilic sols. Examples are rubber, gelatin, gum, starch, etc.
Lyophobic sols require different methods of preparation, such as physical disintegration methods, chemical methods and condensation methods. Examples are metals sols like sulphur, gold, etc. These sols are impure; hence, the purification of sols is a critical step during their preparation process.
Writer – Ajay Shende
Subject Matter Expert (Chemistry)

